Debate is raging over how much individual risk should influence screening and treatment.
Calls for the introduction of risk-based screening are growing louder, but one expert says the current program needs refining rather than retirement.
As the voices questioning whether we can improve on Australia’s population screening become more prominent, factions with differing views are emerging, and the debate about risk-based breast cancer screening is heating up.
Those who support sticking with the current program – where all women between 50 and 74 are encouraged to screen every two years – point to the data: five-year survival rates are going up and mortality rates are going down. But others want a more risk-based approach, saying the current way of doing things is simultaneously oversensitive and not sensitive enough.
Breast cancer is projected to be the most common cancer in Australian women this year, well ahead of melanoma, colorectal cancer and lung cancer in terms of age-standardised incidence rates. One in seven Australian women will develop breast cancer at some point in their life, with the likelihood increasing with age.
Australia’s population-based national breast cancer screening program – BreastScreen Australia – was first introduced in 1991 and became fully functional in 1995. Women aged 50-74 years are specifically invited to undergo a free breast screen (usually a 2D full field digital mammogram) every two years at a state BreastScreen facility, although women aged 40 and over can participate.
The program aims to minimise illness and mortality by detecting cancers before the become symptomatic. Early detection means treatment can start years sooner than if people waited for symptoms to appear.
Screening (mostly) works
The national breast cancer screening program seems to be achieving its objectives. The age-standardised mortality rate for breast cancer has decreased from 16.3 deaths per 100,000 persons in 1995 to 9.8 deaths per 100,000 in 2021, according to data from the National Mortality Database.
“One of the reasons for introducing [the program] was that there hadn’t been a change in mortality from breast cancer for many, many decades. Since [then], we’ve had about a 30% reduction in breast cancer mortality. And it’s due to a combination of early detection and more effective breast cancer treatment,” Professor Mary Rickard, radiologist and medical director at the Sydney Breast Clinic, explains.
In addition to the reductions in mortality rates, the Australian Institute of Health and Welfare estimates the five-year relative survival rate for women with breast cancer has increased from 83.8% in 1995-1999 to 92.0% in 2015-2019.
But while the Australian data shines a positive light on mammography, international data are not always as rosy. A recent controversial meta-analysis of randomised clinical trials, published in JAMA Internal Medicine, reported mammography screening for breast cancer did not save lives by extending an individual’s life (compared to not being screened) based on the results of two trials involving over 70,000 individuals; one from Sweden, the other from the United States.
“This was a new and timely piece of evidence,” says Dr Brooke Nickel (PhD), a research fellow from the University of Sydney.
“It showed that while we know mammography screening does pick up more cancers and has the potential to improve outcomes in some women, there is now a growing body of evidence showing the benefits might not be as great as once thought – and that there are some potential harms that come with screening.”
Screening doesn’t find every breast cancer
The national breast cancer screening program is currently facing two significant challenges.
One is the underdiagnosis of breast cancer.
BreastScreen Australia primarily uses 2-dimensional full field digital mammography to screen asymptomatic individuals, although some centres use 3D mammography (tomosynthesis). But not all cancers can be easily seen on a mammogram. Dense breasts have more fibrous and glandular tissue than fatty tissue. The former shows up as white on a mammogram, masking tumours, which are also white.
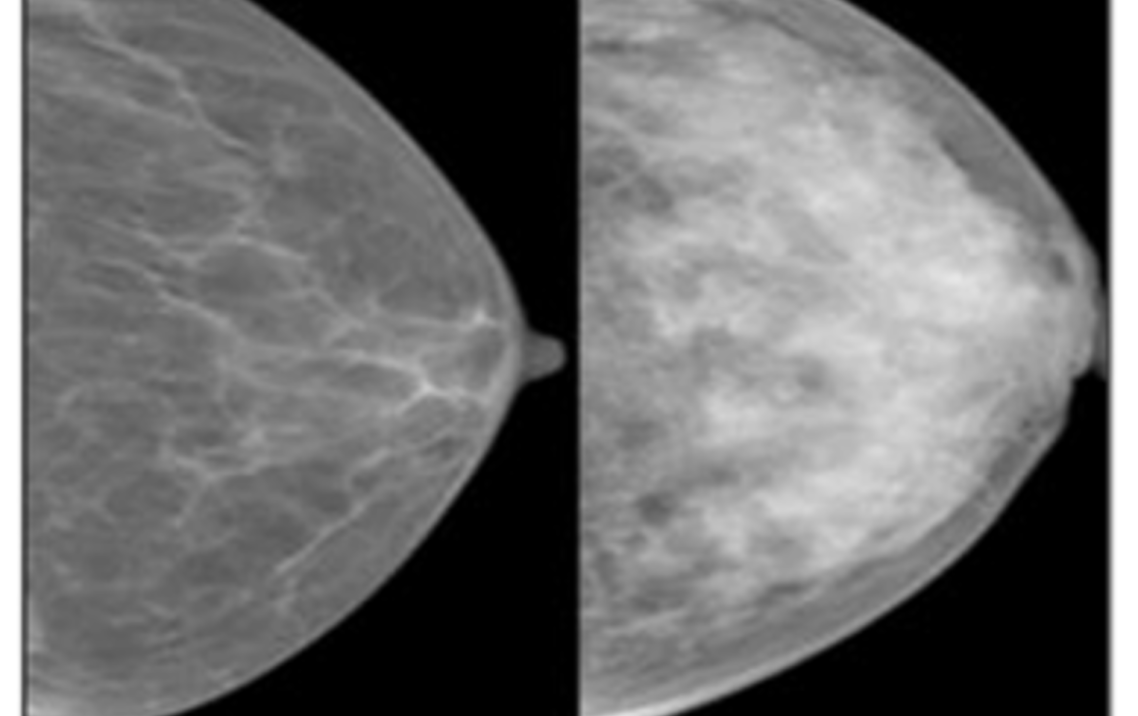
Mammography images of a fatty (left) and dense (right) breast. Source: Breast Cancer Network Australia.
Consequently, women with high density breasts have an 18-fold increase in the risk of developing an interval breast cancer, a symptomatic cancer that develops in the first 12 months after screening is completed, says Professor Rickard.
Breast cancers can also develop from the fibroglandular tissue.
“A big screening study showed when you compare very dense breasts with very fatty breasts, you have about five times the risk of developing cancer in the dense breast,” Professor Rickard explains.
Around 40% of women over the age of 40 have dense breasts. That is a significant proportion of the population who could potentially benefit from incorporating a more risk-based approach into the current screening program, according to Professor Rickard.
Australia is experiencing a rise in breast density advocacy. Currently, only two Australian jurisdictions (Western Australia and South Australia) routinely inform women of their breast density following a mammogram.
BreastScreen Australia does not recommend recording breast density in their 2020 position statement because “there is no agreed consistent and reliable way to measure density or consensus management on how to optimally manage breast density”. Nor do they recommend providing additional testing for women with dense breasts, despite acknowledging “there is evidence that high breast density can mask breast cancer on a mammogram.”
Professor Vivienne Milch, medical advisor to the Commonwealth Department of Health and Aged Care on screening policy, told our sister publication The Medical Republic earlier this year: “We’re aware of the growing momentum of advocacy and also some women’s desire to know their breast density… [and that] different states have either a different policy or are trialling, or piloting.
“It’s very important that we see the results of that research. BreastScreen will keep abreast of the evidence as it’s developing.”
Several studies evaluating risk-based screening for breast cancer, including trials in the United States, Italy and the UK, are at varying stages of completion.
Dr Nickel, together with BreastScreen Queensland and the Sunshine Coast Hospital and Health Service, has recently launched a randomised clinical trial, the first of its kind anywhere, examining how women react and respond to more personalised information about their breast cancer risk.
“Women are really interested in the topic, but we don’t know how hearing this information impacts them in the long term with respect to things like anxiety or confusion, which may dissipate over time,” Dr Nickel tells Oncology Republic.
“Is it better to tell them? Is it not? These are the kinds of questions we are trying to figure out.”
One of the burning issues is what women do with information once they have it, says Dr Nickel.
“We know that having supplementary screening – a mammogram plus an MRI, or a mammogram plus an ultrasound – finds more cancers in women with dense breast tissue.
“But what we don’t know, what we don’t have any good quality evidence about, is whether doing that will improve mortality, morbidity and long-term outcomes for breast cancer in women with dense breast tissue.”
Ensuring equity of access for additional risk-based screening is a significant matter.
Women can go outside the BreastScreen program to be screened in the private setting, at any age and at any interval, with technology that includes digital breast tomography, contrast enhanced mammography, handheld or automated ultrasound and magnetic resonance imaging – should they have the means and capacity to do so.
“It’s all well and good if we tell women, ‘You have dense breasts, go talk to your doctor’ and for the doctor to say, ‘All you can do is get additional screening’, but if it’s not equitable for an individual to [get that screening], they’re left with their hands tied. It’s challenging to know you have this potential risk factor that there’s nothing you can do about,” says Dr Nickel.
Screening also finds too many cancers
The other major challenge breast screening comes up against is overdiagnosis, which occurs when slow-growing or benign cancers that would normally not be diagnosed during a woman’s life are detected during routine screening. This can lead to overtreatment, as all women who are diagnosed with breast cancer are offered treatment.
There is much debate regarding the significance of overdiagnosis, with different perspectives on whether the benefit of screening outweighs the risks. Many point to the possible “cascade of harms” spanning physical, psychological and financial domains, triggered by overdiagnosis1.
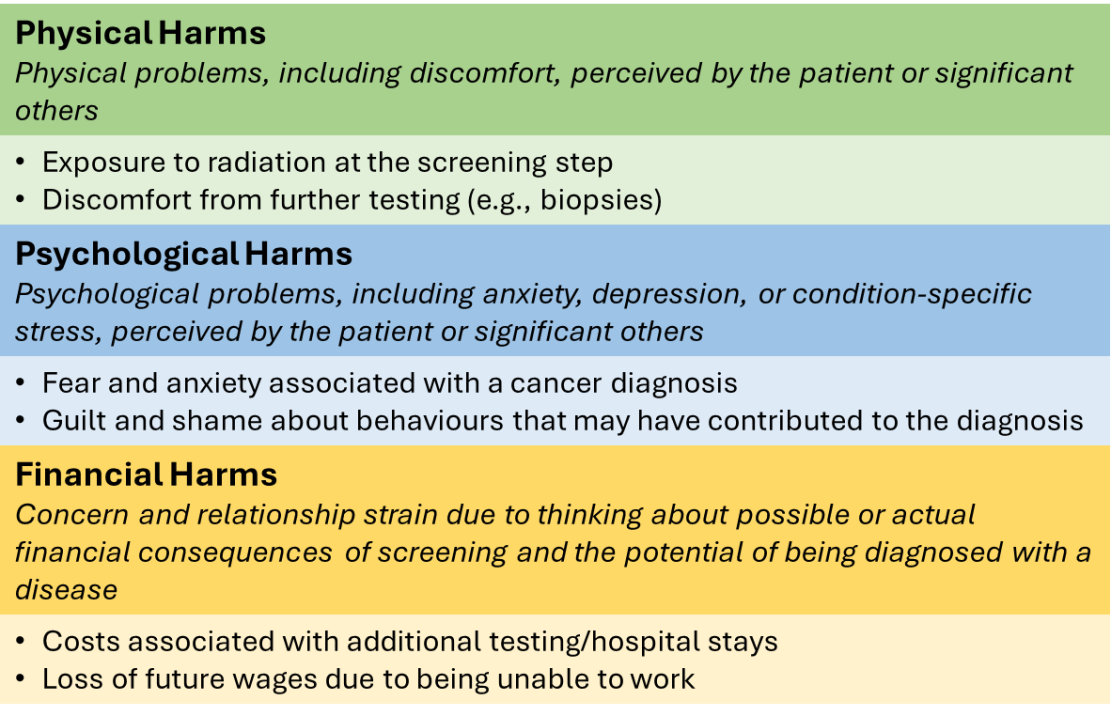
Taxonomy of the harms of cancer screening. Produced from text of Harris et al. 20142.
BreastScreen Australia refers to Cancer Australia’s position statement, endorsed by Cancer Council Australia and the Royal Australian and New Zealand College of Radiologists, when the conversation turns to overdiagnosis.
Last revised in 2014, it acknowledges the possibility that some of the cancers detected during screening may be an “overdiagnosis”, with estimates that for every 1000 women screened, about eight cancers are found and unnecessarily treated (as these cancers would likely not have caused any harm had they remained undetected). For context, overdiagnosed cases in US programs are estimated to comprise around 15%3.
But Cancer Australia’s position statement also makes two other key points:
- Most breast cancers identified through screening programs are ‘progressive’ and would become symptomatic if left untreated, and
- It is currently not possible to determine which screening-detected breast cancers will be problematic and which cancers could have remained undetected without becoming symptomatic.
However, not everyone agrees. Some experts argue that only a very small proportion of cancers go on to become invasive and require treatment.
Knowing which cancers require intervention and which can be left alone is a crucial part of the overdiagnosis debate.
Associate Professor Katy Bell, a clinical epidemiologist from the University of Sydney whose research aims to identify sustainable models of healthcare that maximise benefits while minimising harms, is cautiously optimistic about our ability to determine which cancers will be problematic.
“I don’t think you can ever have complete certainty with any prediction of the future. But the more we know about the natural history of these lesions, the more we are able to make the distinction between the more indolent [end of the] spectrum of cancer versus the clinically aggressive.
“We already have quite a bit of knowledge about prognostic factors that help make that distinction based on clinical and pathology factors. For example, we know women with a low risk ductal in situ carcinoma may be safe to follow up with active surveillance [rather than having] surgery upfront.”
How would risk based screening work?
Risk-based screening still aims to maximise the benefits of screening, using early detection and treatment to reduce breast cancer-related deaths, while simultaneously minimising the harms by reducing potentially unneeded screens and avoiding underdiagnosis and overdiagnosis.
Women are grouped based on their initial screening risk, which determines their individual level of screening and testing moving forward, similar to the way the revised cervical screening pathway operates. This means women with a higher risk of breast cancer, such as those with dense breasts, may be screened more frequently with ultrasound or an MRI, while women with a lower risk may only need to be screened every five years instead of two.
We are closer to risk-based screening than you might think, says Professor Nickson, who leads the Roadmap for Optimising Screening in Australia – Breast Cancer (ROSA) project for Cancer Council Australia.
She says the balance of benefits and harms still favours the population-based approach, provided the participants are aware of both sides of the story. But that doesn’t mean she is against the idea of improving how BreastScreen works – or how we think about it.
“The current screening program is [already] risk-based, because it’s offered to women within a certain age range, and within that there’s annual screening offered to particular subpopulations based on a personal history of breast or ovarian cancer – but these policies vary between states and territories here in Australia,” she says.
“I don’t think of it as stopping the current approach, so much as refining it,” Professor Nickson tells Oncology Republic.
The federally funded ROSA initiative aims to systematically investigate and build evidence and consensus for risk-based cancer screening in Australia in terms of health policy and clinical practice.
It produced its initial four-to-five-year Roadmap towards risk-based breast cancer screening in August 2019, as well as submitting eight technical reports to the DoHAC between 2019 and 2022. The final set of recommendations and the updated Roadmap were presented to DoHAC earlier this year.
Professor Nickson supports the move to inform women of their breast density risk, if there is evidence-based information and consistent approaches in the health system to support women who may be at increased risk of breast cancer.
“I think there is an imperative for BreastScreen to ensure women with higher breast density are informed about options for investigation in relation to breast density as an independent biological breast cancer risk factor and also the masking effect of higher breast density, which can conceal early-stage cancers that might otherwise be picked up by standard mammography screening,” Professor Nickson says.
“This type of approach, if consistent with the available evidence, would constitute a form of risk-based screening.”
Dr Nickel and Professor Rickard also support the inclusion of breast density, though Dr Nickel acknowledges that incorporating variable factors, such as breast density, which changes with age, in algorithms is challenging.
They also want family (genetic) history included in any potential risk-based screening program.
“When should you start to assess risk in women? There’s a lot of literature where people recommend you start to assess women for their risk of breast cancer at an early age to help plan at what age you might start screening. For example, were your mother to have breast cancer at age 40, you should start screening at age 30,” says Professor Rickard.
Professor Rickard also suggests including prior chest irradiation, the use of hormone replacement therapy and lifestyle factors like weight and alcohol consumption.
Professor Bell, who also has expertise in evaluating the clinical effectiveness of healthcare, stresses the need to base any hypothetical risk-based screening approach on outcomes that are important to both clinicians and patients, while not going overboard with including every potential risk factor.
Striking the right balance between collecting enough data to accurately assess a patient’s risk while not requiring them to complete mountains of paperwork will also be a challenge.
“There tends to be a law of diminishing returns, where once you’ve got the important predictors in your model, adding further things will only add a little bit more value.
“Things that you could easily measure, or would always have [on hand], like age and family history, should be your baseline comparator [before] seeing whether the new thing that you’re measuring adds much to the risk prediction model. For example, people are looking at polygenic risk scores, but it’s important to see how much they add to the existing knowledge of risk rather than looking at them on their own,” Professor Bell says.
Meanwhile, in September 2022, the University of Melbourne launched a new NHMRC-funded Centre of Research Excellence: the My Breast cancer RISK Centre (MyBRISK).
The centre will use “artificial intelligence computer techniques to analyse millions of mammograms [to] produce new and more powerful mammogram-based risk factors” and will “create a screening model that can better support younger women, women with family history and Aboriginal and Torres Strait Islander women (who have lower participation and higher mortality rates),” reads the centre’s executive summary.
Ensuring that the tools used to stratify screening populations according to their individual risk are valid and effective in the Australian population is critical, according to Professor Nickson.
There are several available, such as the Breast Cancer Risk Assessment Tool (BRCAT, commonly known as the Gail model), the Tyrer-Cuzick tool (also referred to as the IBIS risk assessment tool), the BRCAPRO and iPrevent. These tools perform differently in different settings, populations and screening programs.
“It’s very important to conduct our own validation studies to see how [they] perform in our own population. I [also] think there is a piece of work around whether we need to use a very detailed questionnaires or tools, or whether we could use a more simplified approach,” she says.
Getting horses to drink
Even if we can identify the best tool to use to determine an individual’s risk of breast cancer and propose new models of care that ensure equitable access to the necessary screening and treatment based on individual risk, there still may be another significant barrier to overcome – getting both the government and public on board with using more of a risk-based approach.
“There’s a question of whether there is a threshold of evidence that would mean everyone involved in implementing a change would just make it happen,” Professor Nickson says.
“We’ve seen that happen in cervical cancer screening. The advent of the HPV vaccination has significantly changed how we can interrupt the natural history of [cervical] cancer. But we don’t have that same singular disruptor in breast cancer screening.”
Early breast cancer screening is still highly valued by Australian women, according to Dr Nickel.
“There’s lots of research – both quantitative and qualitative – showing women truly believe screening will be of benefit. There’s a really high enthusiasm for screening, especially in breast cancer.”
BreastScreen Australia’s National Accreditation Standard aims for a 70% participation rate. Preliminary data from the AIHW reveals about 1.8 million of the 3.6 million women aged 50-74 who were eligible for screening mammograms participated in the program in 2021 and 2022, resulting in a national age-standardised participation rate of 49.4%.
Consistent with those figures, the 2023 BreastScreen Australia monitoring report reveals 50% of all breast cancer cases in women aged 50-74 in 2019 were found through the screening program.
While there was a decline in participation rates between 2019 and 2021 (hello, covid), they had already plateaued somewhat between 2015 and 2019. In 2021/22, women aged 65-69 had the highest age-standardised participation rate (54.5%), 50-54 had the lowest (45.4%). State-wise, Tasmanians turned up in the greatest numbers (57.9%), and the lowest in the Top End (34.0%).
The idea of regular breast examinations and screening mammograms is entrenched in the minds of the public and are renewed each time a celebrity is diagnosed with cancer. This is by no means a bad thing, but it hints at the potential amount of work that will be required to shift away from the established mindset.
“People seem more accepting of being offered more screening if they’re in the high-risk group, but a lot less accepting of having less, or no, screening [if they’re in the lower risk groups]. For the risk-based screening approach to work, you need to have acceptance at both ends. Otherwise, it will end up being a quasi-risk-based approach, where you’re adding more screening to the high-risk people,” says Professor Bell.
Regardless of what changes occur during the potential shift towards a more risk-based screening approach, Professor Nickson stresses the need to avoid a complete departure from the existing program.
“It’s really important to maintain the integrity of the current BreastScreen program as we look towards a potential shift towards risk-based screening. The current program works extremely well and has a high level of accountability that no other breast cancer surveillance or diagnostic service across the country has. It’s already very scrutinised and intensely evaluated, but that creates a strong foundation to refine the program and then make sure it’s improving as expected.
“There is also evidence that access to the current program varies according to socioeconomic status and other demographic factors, highlighting the importance of ensuring established technologies benefit all populations equitably while we adapt to new technologies.
“We need to find ways to ensure BreastScreen can provide those services either directly or through engagement or an arrangement with other clinical services where all outcomes continue to be monitored and evaluated as part of the BreastScreen program.”


