Researchers provide proof that targeted IDH1 therapy can alter tumour biology, marking a major step forward in LGG treatment.
Victorian brain cancer researchers have mapped how a new drug can suppress low-grade glioma activity, marking a world-first in clinical innovation.
The study is the first clinical trial conducted through the Brain Perioperative platform, or “BrainPOP,” led by The Brain Cancer Centre and funded by the Victorian Government.
The slow-growing LGGs significantly impact the lives of patients, many of whom are young adults in their prime. Characterised by a specific mutation in a gene called IDH, current treatments are limited and LGGs have long been considered incurable.
The trial tested safusidenib, an oral inhibitor targeting the mutated IDH1 gene, on LGG tumour samples before and after treatment.
Led by researchers from the Royal Melbourne Hospital (RMH), WEHI and the Peter MacCallum Cancer Centre, the study provides direct evidence of the drug’s effect on tumour biology.
Results from this proof-of-principle study, recently published in Nature Medicine, demonstrate the potential of safusidenib to alter tumour activity, marking a significant step forward in precision therapy for LGG.
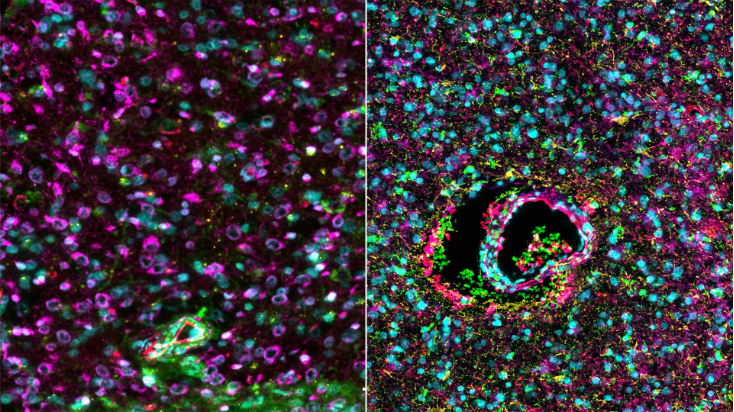
“By combining multi-omic techniques on paired samples from a perioperative clinical trial, we examined direct drug mechanisms of mIDH inhibitor treatment in mIDH1 gliomas,” the authors wrote.
“Confirming earlier results, we show that safusidenib is safe and well tolerated and that the perioperative design is feasible and safe, meeting the primary study endpoint.”
Professor Kate Drummond, director of neurosurgery at the RMH and the trial’s lead investigator, said the response from patients had been overwhelmingly positive, with many excited to be part of the innovative trial, “even though the trial required two operations and intensive treatment”.
“Brain cancer patients are desperate for new treatments, and clinical trials like this are exactly what is needed,” she said.
Professor Drummond said the researchers were driven to ensure Victorian brain tumour patients “have care that is equal to anywhere in the world”.
“This trial is not only a revolution in the way we test new treatments but brings new opportunities for this most deserving group of patients with a devastating disease,” she said.

Dr Jim Whittle, a medical oncologist specialising in neuro-oncology at Peter Mac, and laboratory head at The Brain Cancer Centre and WEHI, said perioperative clinical trials were regularly used in other cancers to understand the true effect of new and emerging treatments.
“These types of trials are vital for advancing drug development but, with the complex and sensitive nature of neurosurgeries, this approach has not previously been used in brain cancer,” he said.
“This new study reveals the power of BrainPOP as a safe and effective platform for accelerating our understanding of new treatments and their real-world impact in the brain.
“For the first time, we’ve seen what a drug is doing in the brain with incredible detail, helping us to clearly identify the next steps for personalising treatment and predicting who would most benefit.”
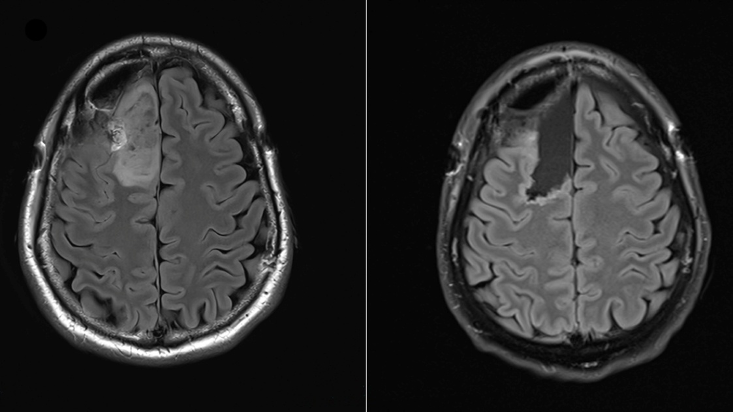
The trial was supported by a $16m investment from the Victorian Government and involved patients who had not yet undergone radiation or chemotherapy. Trial participants took the drug prior to any other cancer treatment – a world first for IDH inhibitors.
The trial was designed to assess the activity of this novel treatment within the brain, and it is too soon to know if, ultimately, these medications will improve outcomes or extend lives for these patients.
Plans are now underway for pivotal studies of safusidenib in diffuse IDH1 mutant gliomas.
“We are deeply grateful for the Victorian Government’s support for the BrainPOP platform, which is vital to our ultimate goal of radically transforming outcomes for patients with brain cancer,” Dr Whittle said.
The collaborative trial program led by The Brain Cancer Centre included the expertise of researchers and clinicians across the Melbourne Biomedical Precinct including Murdoch Children’s Research Institute, Peter MacCallum Cancer Centre, The Royal Children’s Hospital, the University of Melbourne, the Royal Melbourne Hospital and WEHI.
Further trials using the BrainPOP platform are already in development. Patients that meet the entry criteria are offered to join the trials by their treating practitioners at participating hospitals.
Founded by Carrie’s Beanies 4 Brain Cancer, The Brain Cancer Centre was established in 2021 in partnership with WEHI and with support from the Victorian Government.


